- метаболические препараты не имеют побочных эффектов и не вызывают осложнений
- высококвалифицированные специалисты с опытом до 30 лет
- центр работает с 1993 года
Что мы лечим?
Как проводится лечение?
Метаболическая терапия предполагает амбулаторное лечение. Это значит, что пациенты периодически (1 раз в 5-7 дней) осуществляют визит к врачу для осмотра и коррекции лечения, поскольку изменения в состоянии больного под влиянием Метаболической терапии происходят достаточно быстро.
Курс лечения при разных заболеваниях варьирует от 10-12 дней (например, лечение геморроя) или 60-80 дней (лечение детского церебрального паралича, задержки психоречевого развития).
Медицинский центр "ПРИМАВЕРА МЕДИКА" им. А. П. Хохлова
Понедельник-суббота с 09:30 до 17:30
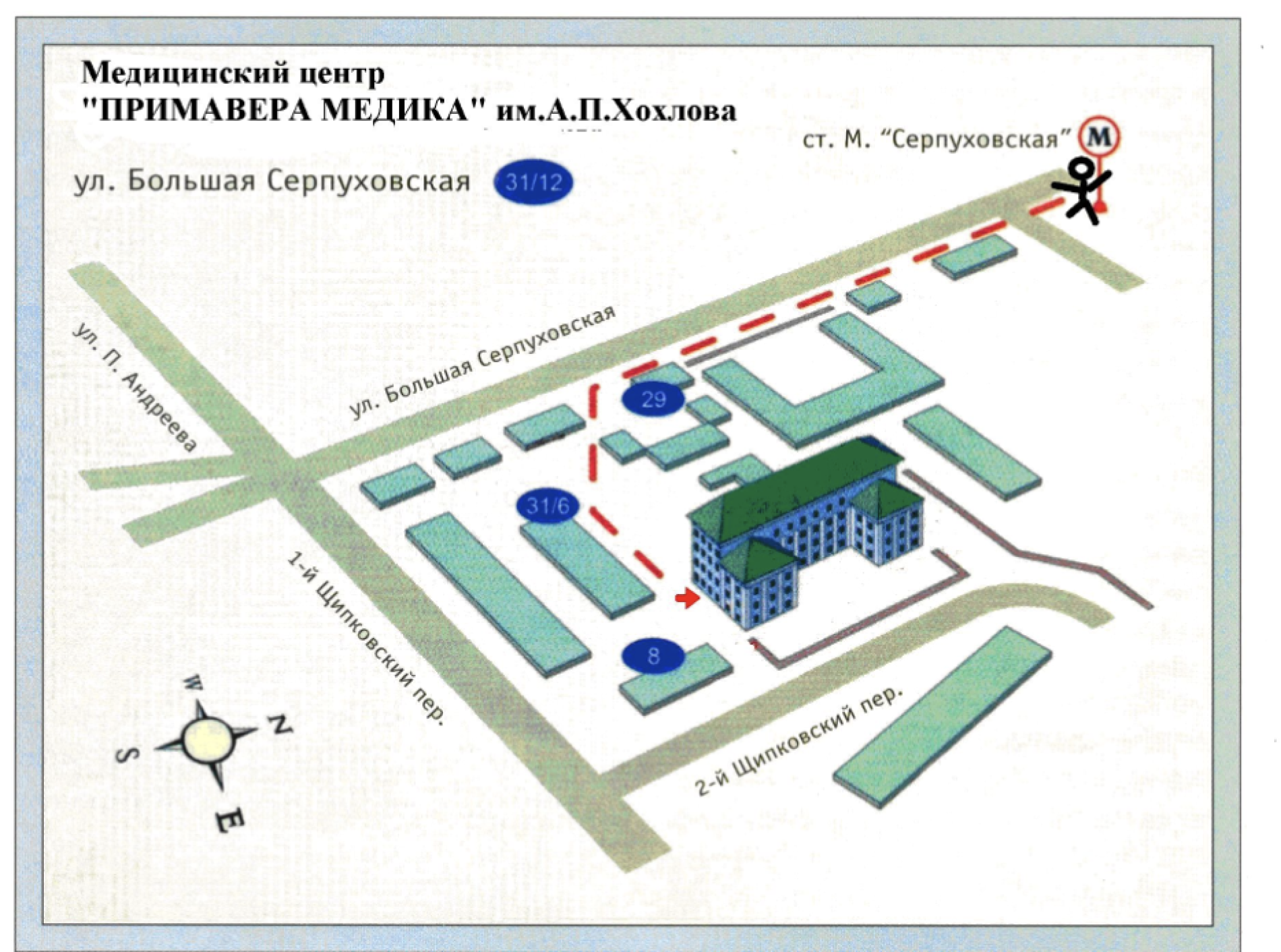
- 115093, Москва, ул. Б. Серпуховская, д. 31, корп. 12.
-
+7 (499) 236-71-22
+7 (499) 236-50-64
+7 (499) 237-94-49
What"sApp: +7 (916) 801-84-68 - info@primavera.ru